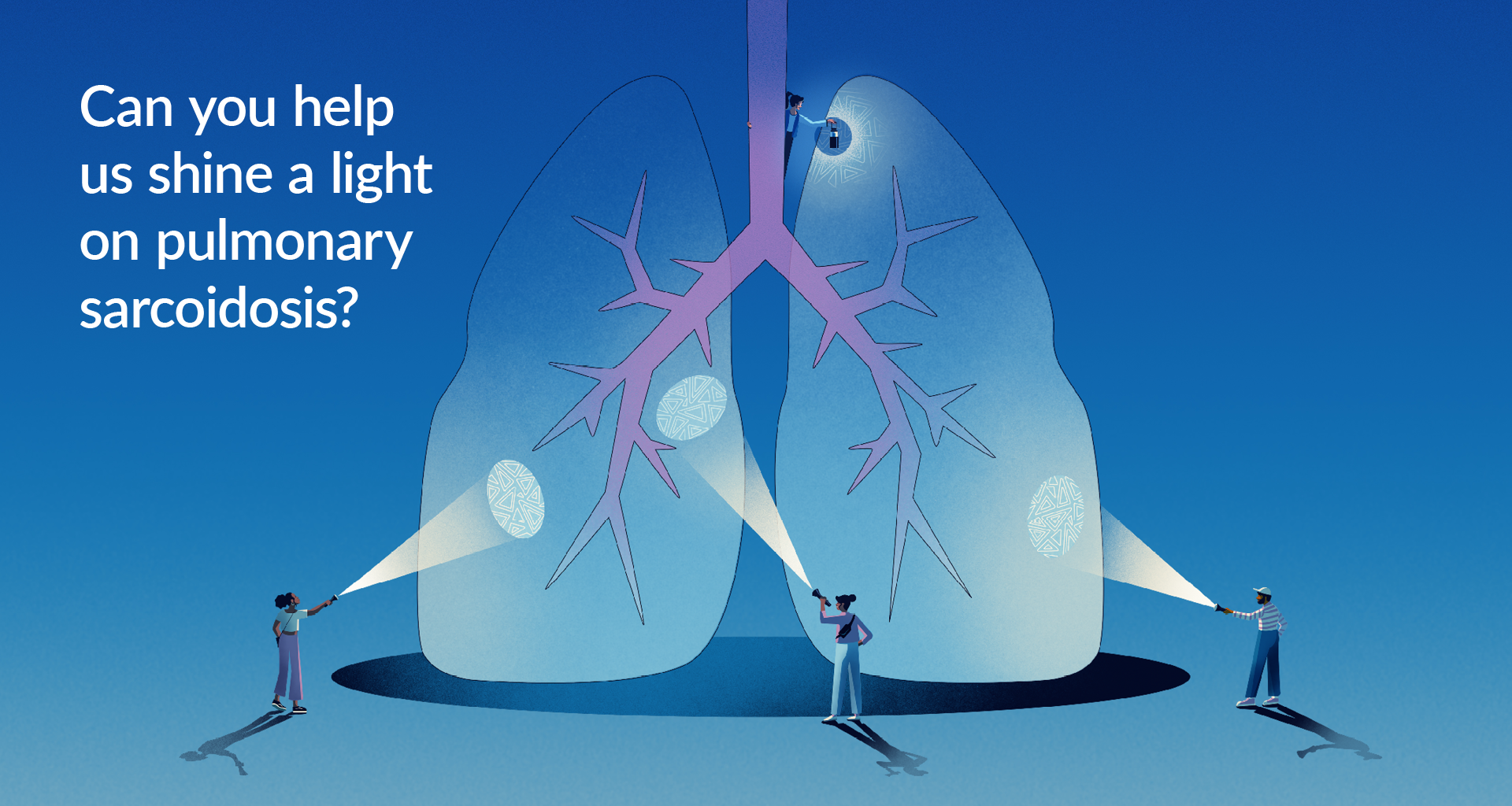
Adjustments to the criteria associated with pulmonary hypertension, cardiac sarcoidosis, and BMI have been made for this clinical trial. Even if you were not eligible to participate in this study before, you may be eligible now!
We encourage all patients, including those who have previously filled this survey out, to click the button below to see if you qualify! For US Audiences Only.

Purpose
Novartis has partnered with the Foundation for Sarcoidosis Research for an exciting clinical trial. The purpose of this trial is to investigate a potential new drug, CMK389, which hopes to improve the ability of the lungs to work for patients with chronic pulmonary sarcoidosis.
Who can participate in this study?
Potential participants should fulfill the following criteria:
-
-
- Be between the ages of 18 and 75 years
- Have a body mass index (BMI) within the range of 18-46 kg/m2 (UPDATED CRITERIA)
- Have biopsy proven pulmonary sarcoidosis diagnosed at least a year ago
- Be receiving 5-15 mg/day of prednisone (or equivalent steroid medications) for > 6 consecutive months
- Be receiving methotrexate or azathioprine for > 6 consecutive months prior to screening (i.e. the visit at which you are assessed to see if you qualify to participate in the study)
-
Potential participants who fulfill any of the following criteria may not be able to participate:
- Diagnosis of significant pulmonary hypertension requiring treatment
- Active cardiac sarcoidosis requiring treatment (UPDATED CRITERIA)
- Known diagnosis of neurosarcoidosis
- Patients with pulmonary arterial hypertension ***Patients with systemic hypertension (i.e. high blood pressure) may be eligible for participation (CLARIFICATION)
- Prior treatment with rituximab (Truxima®, Rituxan®) , canakinumab (Ilaris®), anakinra (Kineret®), and tocilizumab (Actemra®)
- Current smokers, including but not limited to tobacco, marijuana products, electronic cigarettes, or vaping device (respiratory inhalers or nebulizers for delivery of prescribed medication for pulmonary sarcoidosis are allowed)
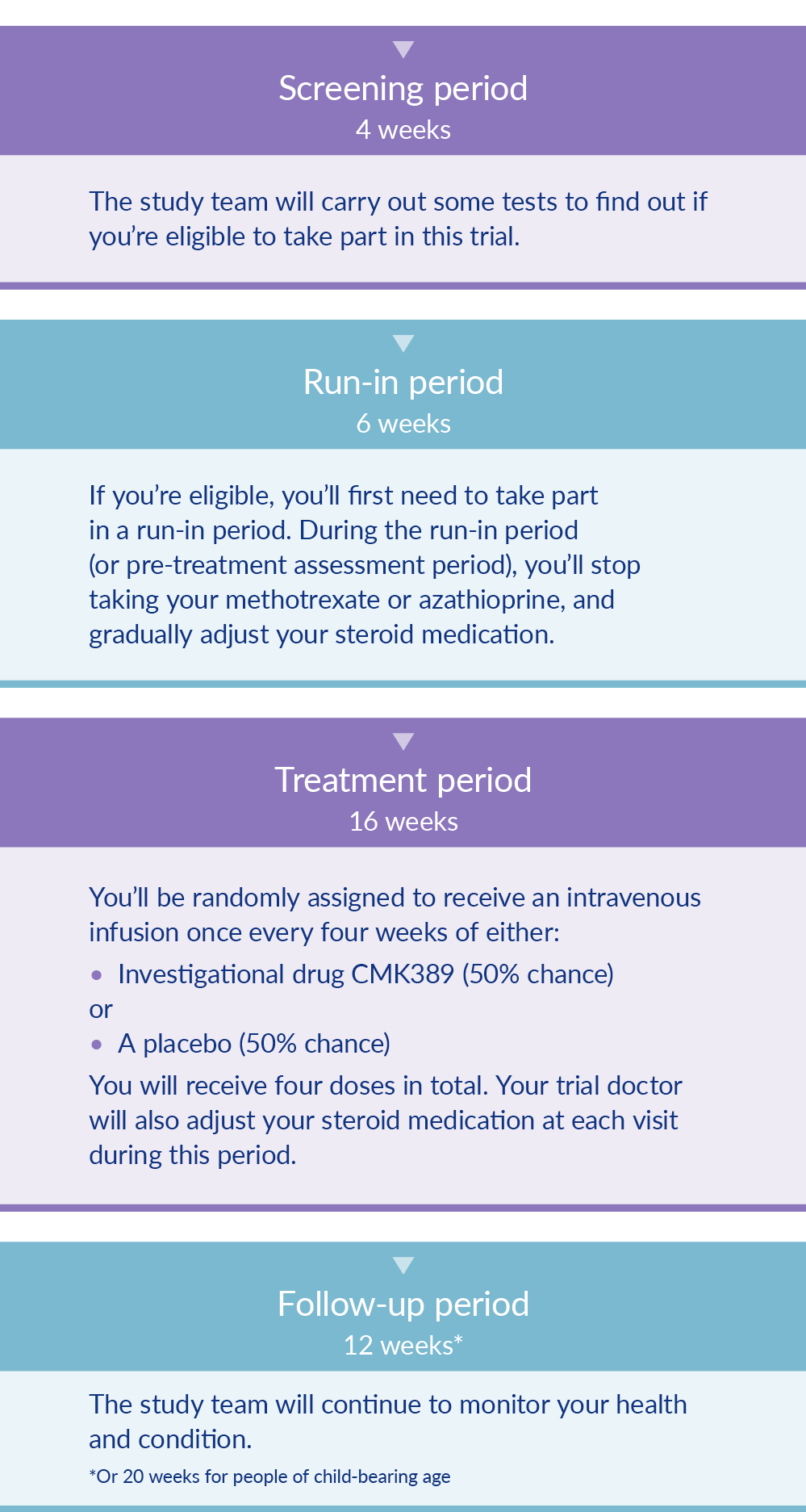
Study Design
Eligible participants in this study will either receive the study drug or a placebo through intravenous infusion (i.e. administered into a vein) every 4 weeks. A total of 4 doses will be given. A placebo looks just like the study drug but does not contain any active medication. As a double-blind study, neither participants nor investigators will know which patients receive CMK389 and which receive the placebo
What do Clinical Status Evaluations Typically Consist of?
A typical evaluation will include a 6-minute walk test, spirometry assessment, and a questionnaire. These tests serve as a way to ensure participant safety as well as mark clinical status.
What are Placebos Used for?
Placebos are necessary for ensuring that any treatment effects seen during the study are truly due to the study drug and not an external factor.
Why are Participants Randomly Selected for the Study Drug?
Randomization is used to avoid bias when assigning participants to their treatment group.
Why is this study double blinded?
Similar to randomization, blinding helps avoid (conscious and unconscious) biases that could potentially arise when aware of treatment assignments.
Active Study Locations
- Mount Sinai Hospital (New York City, New York)
- National Jewish Health (Denver, Colorado)
- Northwestern University (Chicago, Illinois)
- University of Florida (Gainesville, Florida)
- University of Pittsburgh (Pittsburgh, Pennsylvania)
- East Carolina University (Greenville, North Carolina)
- University of Alabama (Birmingham, Alabama)
- Jefferson Health (Philadelphia, Pennsylvania)
- Johns Hopkins University (Baltimore, Maryland)
- Additional locations may be added
Other Locations
- University of Kansas is only accepting patients through direct contact, to learn more about how to participate at this site, click here
- Additional locations may be added
