Authors: Arindam Singha, MD1; Tricha Shivas, MBe2 ; Elliot D. Crouser, MD1

Sarcoidosis patients are often treated with immune suppressing drugs that further increase susceptibility to COVID-19 infection. Hence, healthcare providers and other patient advocates are highly motivated to enlist measures to mitigate the risk of serious COVID-19 infection in this patient population.
The COVID-19 era has taken a catastrophic toll in terms of human lives lost on a global scale, however, the pandemic has been met with one towering success. Several novel vaccines, such as the message RNA (mRNA) based vaccines developed by Pfizer-BioNTech and Moderna that have received the Emergency Use Authorization (EUA) by the Food and Drug Administration (FDA), are shown to be highly effective and well tolerated by the vast majority of patients. Despite the unprecedented rapid development of highly efficacious vaccines, there are major barriers to delivering vaccines to those who are most likely to benefit.
Valuable insights explaining the failure to effectively vaccinate sarcoidosis patients are provided from a recent survey conducted by the Foundation for Sarcoidosis Research (FSR). The survey was designed to determine the rate of vaccinations among patients with sarcoidosis, vaccine tolerance, to elicit the major reservations to being vaccinated among those who choose not to be vaccinated, and to ascertain the reasons that vaccines were not delivered to those who were motivated to be vaccinated. A brief 8 question survey was sent via email and social media posts (Twitter and Facebook) to the members of a sarcoidosis online support community that is sponsored by FSR. A total of 262 (of approximately 5000 total) members responded to the survey in February 2021.
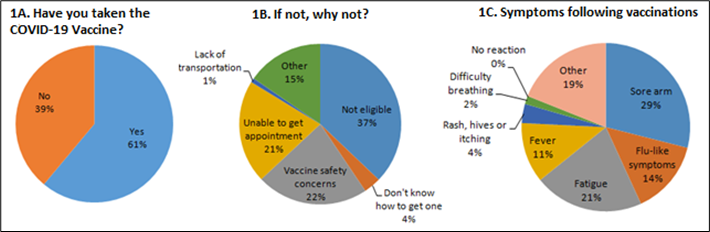
At the time of the survey, 61% of the responders had at least one dose of either the Pfizer- BioNTech, or the Moderna vaccine (J&J vaccine was yet to be granted EUA at the time of the survey). Of the 39% of responders who had not received the COVID-19 vaccine, a majority were judged to be ineligible for the vaccine in their state. However, a significant proportion (25%) cited concerns about vaccine safety as the reason for not getting the vaccine. See Figure 1 for details. Among those who were vaccinated, approximately 44% reported having an adverse reaction, mostly categorized as mild (e.g., local arm pain, fatigue, myalgias, and infrequently fevers), whereas a significant minority of approximately 15% experienced a vaccine reaction characterized as moderate to severe. Namely, approximately 10% of responders reported hives and slightly more than 4% reported respiratory distress for which medical attention was deemed necessary (i.e., a serious adverse event). To put this into perspective, both the Pfizer-BioNTech and Moderna vaccines showed excellent safety profile during the clinical trials conducted in representative human populations, with the Pfizer- BioNTech study reporting a much lower incidence of any severe adverse event of 1.1% [4,5]. Thus, the results of the survey suggest that patients with sarcoidosis are prone to developing a serious, albeit non-fatal adverse reaction following the COVID-19 vaccines. Traditionally, the rate of adverse events in sarcoidosis patients with other vaccines such as the yearly influenza vaccine has been similar to their healthy counterparts [6]. However, whether the safety profile of novel mRNA based COVID-19 vaccines is different in sarcoidosis remains to be determined. Even if this is the case, the risk of non-fatal, self-limited adverse vaccine reactions should be weighed against the reported risk of potential life-threatening COVID-19 infection in sarcoidosis and other ILD patients (1-3).
There are a number of limitations to our study. Given the small sample size of our survey, and the low response rate, the survey results may be biased (e.g., those with vaccine side-effects may be motivated to report them). Since the completion of the survey, many states have expanded their vaccine availability to all individuals above the age of 16, thereby addressing some of the reported concerns relating to vaccine access. Nevertheless, healthcare providers should be aware that approximately 25% of those who did not receive the vaccine chose not to receive it citing concerns about vaccine safety and another 25% could not get a vaccine due to lack of transportation, inability to secure an appointment for the vaccine or simply not knowing how to get one. Some of these issues relating to vaccine access may be unique to the often underprivileged sarcoidosis patient population (7). This survey highlights the barriers to vaccination beyond the eligibility that still exists in the country.
Figure 1 A-C. Responses to three out of eight questions from the survey. Total number of responders = 262.
Read more about sarcoidosis and the COVID-19 pandemic.
Affiliations:
1Division of Pulmonary and Critical Care Medicine, The Ohio State University Wexner Medical Center. Dr. Elliott Crouser is chair of FSR’s Scientific Advisory Committee.
2 Foundation for Sarcoidosis Research
References
- Southern BD. Patients with interstitial lung disease and pulmonary sarcoidosis are at high risk for severe illness related to COVID-19. Cleve Clin J Med 2020; Jun 18; doi: 10.3949/ccjm.87a.ccc026.
- Morgenthau AS, Levin MA, Freeman R, Reich DL, Klang E. Moderate or Severe Impairment in Pulmonary Function is Associated with Mortality in Sarcoidosis Patients Infected with SARS-CoV-2. Lung 2020; 198(5):771-775.
- Baughman RP, Lower EE, Buchanan M, et al. Risk and outcome of COVID-19 infection in sarcoidosis patients: results of a self-reporting questionnaire. Sarcoidosis Vasc Diffuse Lung Dis. 2020;37(4):e2020009. doi:10.36141/svdld.v37i4.10726
- Anderson EJ, Rouphael NG, Widge AT, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383(25):2427-2438. doi:10.1056/NEJMoa2028436
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
- Tavana S, Argani H, Gholamin S, et al. Influenza vaccination in patients with pulmonary sarcoidosis: efficacy and safety. Influenza Other Respir Viruses. 2012;6(2):136-141. doi:10.1111/j.1750-2659.2011.00290.x
- Gerke AK, Judson MA, Cozier YC, Culver DA, Koth LL. Disease Burden and Variability in Sarcoidosis. Ann Am Thorac Soc. 2017;14(Supplement_6):S421-S428. doi:10.1513/AnnalsATS.201707-564OT