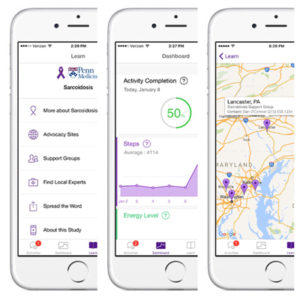
As we announced earlier this month, Penn Medicine launched the first ever Apple Researchkit app for sarcoidosis patients. Spearheaded by Dr. Misha Rosenbach, a member of FSR’s Scientific Advisory Board, the app aims to not only provide patients across the country (and hopefully globe!) with instant access to disease-specific resources on their phones, but also to connect researchers to this effort by providing more detailed insight on the daily lives of sarcoidosis patients. If patients choose to opt-in to certain features of the app, their data tracked in the app can be reported to a research database that, in addition to optional monthly surveys, allow researchers to track patterns of the daily burdens that patients face. A review of the app on Eureka Alert notes:
“Among potential queries the technology will allow investigators to explore: When sarcoidosis is flaring, are patients walking fewer steps? Do they miss work? Does their disease flare after a week’s worth of sunny days? Does geographic location affect symptoms? Is there a seasonal variability? How quickly do patients respond to treatments?”
Another review was written by Johns Hopkins cardiologist Satish Misra, who also is a founding partner and editor of iMedicalApps. He praised the app’s ingenuity in recruiting rare disease patients- typically a tough population to gather massive information on because of the rarity of the disease and lack of physicians with a deep understanding of the disease. While he is slightly skeptical about the longevity of the app’s success based upon fading user engagement from other Researchkit apps like “Asthma Health,” he remains optimistic because “things are different with a rare disease, where the sense of community among patients may be stronger.”
Daniel O’Connor, the fourth-year med student who developed the app with Dr. Rosenbach, says they hope to make this the largest study on sarcoidosis ever, with the help of technology. By using a mobile platform, they have the potential to reach and follow up with more patients than any clinical trial before. Already they’ve had over 500 downloads of the app, half of whom have completed the entire process of giving informed-consent and are taking regular surveys that are sent back to Penn Medicine. This app may only take a few months to recruit the number of patients that the largest studies have taken years to round up. Dan says it’s too early to say if and when an Android version of the app will be developed. They’ve received many emails and know it could greatly expand their outreach. The current app uses Apple’s Researchkit platform, which may make it more difficult to simply adapt the existing app for Android use. However, there are currently several Android platforms that are trying to mimic Reasearchkit’s capabilities. If user engagement stays up, the researchers will have reason to expand the app so it’s accessible from all mobile devices.
